- Internal Tibial Rotation Exercises for Hypermobility: Improve Knee & Ankle Stability - 20 November 2025
- What Causes chronic pain in Fibromyalgia and Hypermobility - 27 June 2025
- Mast Cells and Fibromyalgia: The Hidden Connection - 19 June 2025
This article covers:
ToggleWhen Fibromyalgia Doesn’t Add Up
If you’ve been diagnosed with fibromyalgia, then chances are you’ve done the rounds. GP, Rheumatology, maybe even Neurology. You’ve heard the words “central sensitisation,” you’ve tried the medications, and maybe you’ve even looked into the idea that it’s all just a glitch in how your brain processes pain.
But what if that’s not the full picture?
What if fibromyalgia isn’t just about nerves being overly sensitive? What if the problem doesn’t start in the brain at all, but in the immune system? or if it is at least, part of the bigger picture.
It’s completely understandable to feel confused or even sceptical. After all, for decades fibromyalgia has been explained almost entirely through the lens of the nervous system. But emerging research is starting to change that. Studies are now showing a potential link between fibromyalgia and the immune system’s first line of defence, mast cells(1).
That said, this isn’t about jumping to new conclusions. It’s about following the evidence. And that evidence is building.
We now have studies showing that mast cells in the brain, particularly in the thalamus, may play a key role in the kind of deep, aching pain fibromyalgia patients experience daily(2). Other research has shown that antibodies from fibromyalgia patients can actually trigger mast cell activation in animal models, leading to fibro-like symptoms in mice(3). It’s the kind of breakthrough that turns heads. Not just because it’s new, but because it fits the lived experience of so many people who feel like their symptoms don’t match up with the explanations they’ve been given.
So, in this blog, we’re going to dive deep.
You’ll learn what mast cells are, how they might be involved in fibromyalgia, and most importantly, what this means for your treatment options. Whether you’ve been living with fibro for years, or you’re newly diagnosed and trying to make sense of it all, this is the place to start.Let’s take a look at what the science actually says and why this could be the beginning of a whole new conversation about fibromyalgia.
What Are Mast Cells and Why Do They Matter?
You’ve probably heard of mast cells in the context of allergies. They’re the ones responsible for releasing histamine when you come into contact with pollen, certain foods, or a wasp sting. However, their role in the body goes far beyond allergic reactions. In fact, they may be one of the most misunderstood and underappreciated parts of the immune system.
Mast cells are found in almost every tissue of the body, especially in areas that act as first lines of defence, like the skin, lungs, digestive tract, and around blood vessels. They’re packed with chemical messengers that can be released in response to various triggers. These include histamine, tryptase, interleukins like IL-6, and tumour necrosis factor-alpha, as well as neuropeptides such as substance P. When released, these chemicals don’t just fight off pathogens. They also create inflammation, influence nerve activity, and can sensitise the nervous system to pain(4).
When mast cells behave normally, this response is helpful. It protects us from infection and injury. But when mast cells become overly sensitive or activated at the wrong times, they can create chaos throughout the body. This is where Mast Cell Activation Syndrome, or MCAS, comes into play.
MCAS is a condition in which mast cells react too easily or inappropriately to triggers. These can be physical, chemical, hormonal, or even emotional. Unlike mastocytosis, which involves too many mast cells, MCAS typically involves normal numbers of mast cells that behave abnormally. This can lead to a whole host of symptoms, including flushing, itching, fatigue, gastrointestinal upset, brain fog, headaches, and even cardiovascular changes like dizziness or palpitations(5).
One of the most important discoveries in recent years has been the identification of the MRGPRX2 receptor. This receptor which is found on the surface of mast cells, can be activated by neuropeptides like substance P. Once activated, the mast cells rapidly release their stored chemicals into nearby tissue. This process, called degranulation, causes widespread effects including pain and inflammation without needing a classic allergic reaction(6).
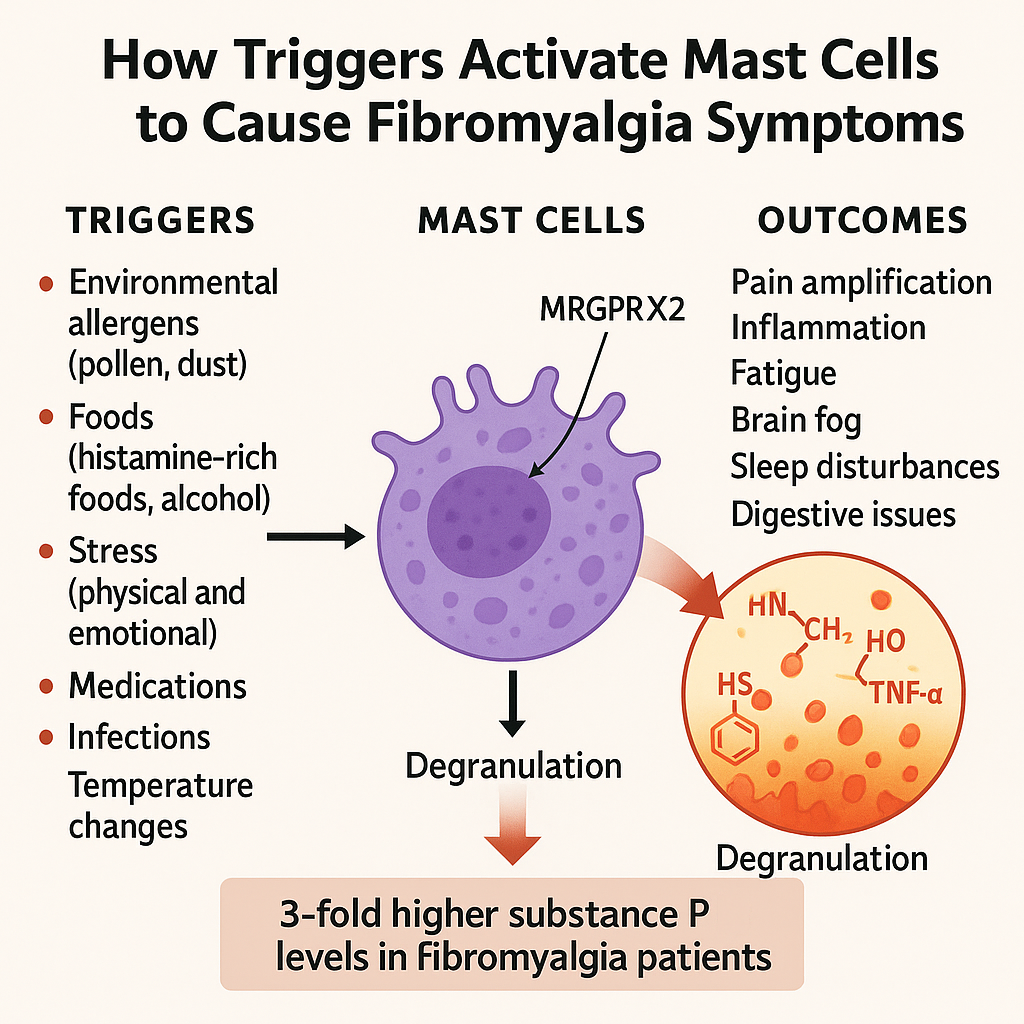
This is particularly relevant for fibromyalgia. A 2025 study found that a drug called modafinil reduced fibromyalgia like symptoms in animal models by blocking this mast cell pathway. The study showed that modafinil inhibited mast cell and microglial activation through what’s known as the SP/MRGPRX2/IL-17 pathway(7). Not only did this reduce inflammation, but it also had a clear impact on pain sensitivity and fatigue in the animals being studied.
So, why does this matter?
Because if mast cells are contributing to fibromyalgia symptoms, as this and other studies suggest, then we are no longer just looking at a problem of pain perception. We’re looking at an immune system problem as well. And if that’s the case, then the standard fibromyalgia toolkit antidepressants, painkillers, and cognitive therapy may be missing the mark entirely for a subset of patients.
It also gives us a way to make sense of the confusing mix of symptoms that so many people report. Flushing, diarrhoea, pressure headaches, rashes, brain fog, low blood pressure, these have all been noted in both fibromyalgia and MCAS, and for years they’ve often been dismissed as unrelated. Now we’re beginning to see how they might all be connected.
The Mast Cell Fibromyalgia Link: A Scientific Deep Dive
If mast cells can misfire and cause widespread symptoms, the next question becomes more direct. Are they actually doing this in fibromyalgia? And more importantly, do we have evidence to support it?
The answer, increasingly, is yes.
One of the most pivotal studies to date examined brain tissue and found a high concentration of mast cells in the thalamus. This region plays a key role in how we process sensory input and pain. These mast cells weren’t inactive. They were actively releasing chemicals like histamine, IL-1β, TNF, and substance P, all of which are known to increase inflammation and nerve sensitivity(2). This begins to explain why fibromyalgia pain is so diffuse and difficult to treat. It may not be a misfiring brain, but rather an inflamed one.
That finding alone is compelling, but another 2025 study provided even stronger proof. Researchers extracted IgG antibodies from people with fibromyalgia and transferred them into mice. Within days, the animals showed clear signs of fibromyalgia. Their pain thresholds dropped, their movement decreased, and mast cell activity rose sharply in their tissues(3). What’s more, this activation happened through the MRGPRX2 receptor, the same one known to be triggered by substance P.
These findings were not isolated. In reserpine-induced fibromyalgia models, rats developed pain, cold sensitivity, and muscle fatigue all symptoms commonly reported by fibromyalgia patients. When the animals were given mast cell stabilisers like ketotifen fumarate, their symptoms improved. Another compound that depleted mast cell mediators also prevented many of the typical fibromyalgia features from emerging in the first place(8). The link between mast cell activity and fibromyalgia symptoms is no longer hypothetical. It’s reproducible.
One of the chemicals at the centre of this process is substance P. Found in significantly higher levels in both the cerebrospinal fluid and serum of fibromyalgia patients, substance P acts as a powerful mast cell trigger(4). Once activated, those mast cells release more inflammatory messengers, which in turn activate more nerves. This creates a self-perpetuating loop, where pain leads to more mast cell activation, which leads to more inflammation, and even more pain.
This loop mast cells in the body influencing the brain’s sensitivity to pain gives us a different way to view fibromyalgia. It’s not just central sensitisation on its own. It may be a two way conversation between the immune and nervous systems. Mast cells activate in the periphery, send their signals, and the brain responds by ramping up sensitivity.
It also helps make sense of something patients have been saying for years. That their symptoms flare during illness, stress, hormonal shifts, or after eating certain foods. These are all common triggers for mast cell activation. What was once labelled as vague or psychosomatic may now be understood as biologically consistent.
And for many people, that realisation is the beginning of something important. A move away from being told to just manage their symptoms, and toward an explanation rooted in science.
Neuroinflammation: The Brain Connection
We’ve talked about how mast cells may initiate the fire, but the brain often determines how far it spreads. For many with fibromyalgia, the pain doesn’t stay peripheral. It becomes amplified, more constant, and harder to trace. This is where the concept of neuroinflammation becomes important.
Modern imaging techniques have allowed researchers to peer inside the brains of people with fibromyalgia. Using PET scans tagged with specific markers, they’ve consistently found signs of activated microglia the brain’s own immune cells especially in regions responsible for processing pain, emotion, and fatigue(9). These microglia, once triggered, begin producing a range of pro-inflammatory cytokines.
This persistent inflammation doesn’t just influence pain. It’s also been linked to fatigue, poor sleep, and cognitive issues. Brain fog isn’t a mystery symptom. It’s likely a direct result of a brain struggling to maintain homeostasis under inflammatory pressure(10).
Importantly, these changes don’t occur in isolation. Several studies have shown that fibromyalgia patients have a unique immune signature one that includes elevated levels of both pro inflammatory cytokines like TNF-alpha and IL-6, and anti-inflammatory markers like IL-10(11). This points to a kind of immune confusion, not simply an overactive response.
And it’s not just the brain. There’s growing evidence for a gut-brain-mast cell axis, where imbalances in the microbiome influence mast cell behaviour, which in turn can impact brain inflammation and mood. While still a developing field, the early data suggest that for some individuals, addressing gut health may directly influence central sensitivity and pain perception(12). While it doesn’t solve the issue, it can reduce the severity.
All of this reinforces a simple but important point. Fibromyalgia isn’t imagined. It’s not just stress, or poor sleep, or low mood. It is, at least in part, a condition rooted in the immune system and how that system interacts with the brain. And that opens the door to treatment strategies that go far beyond managing symptoms.
For many, understanding this connection brings relief. Not because it fixes everything overnight, but because it provides a path forward. A way to understand why the usual approaches haven’t worked. And most importantly, a reminder that what you’re feeling is real and increasingly, explainable.
Symptoms That Don’t Seem to Fit Until Now
Many people with fibromyalgia report symptoms that never quite made it into the diagnostic criteria. Dizziness. Skin rashes. Flushing. Gastrointestinal issues. For years, these have been chalked up as separate problems, or worse, dismissed entirely. But when you factor in mast cell involvement, these symptoms start to look a lot less random.
Histamine intolerance is a good example. This isn’t a true allergy, but rather a sensitivity to foods and chemicals that cause a rise in histamine levels. Symptoms can include headaches, facial flushing, bloating, heart palpitations, and nausea. For someone with underlying mast cell dysregulation, these reactions can happen frequently and without a clear trigger. They often overlap with what many people experience during a fibromyalgia flare(13).
It doesn’t stop there. A number of patients with fibromyalgia also meet the criteria for hypermobility disorders, including Ehlers-Danlos syndrome. Loose joints, easy bruising, stretchy skin, and unexplained pain are common complaints in both groups. While the exact connection is still being explored, researchers have noted that connective tissue fragility may create a permissive environment for mast cell activation, especially around nerves and blood vessels(14). In simple terms, the body is more reactive, and the immune system responds to that instability.
Add in the cognitive issues such as problems with memory, word finding, and concentration, and it becomes clear that fibromyalgia affects far more than just pain. These symptoms were often considered secondary, but they may be just as central to the condition. Microglial activation in the brain and chronic immune signalling may both contribute to this pattern(10). It’s not about poor sleep or anxiety. It’s about inflammation affecting function.
There’s also emerging evidence that fibromyalgia shares biological features with conditions like chronic fatigue syndrome, irritable bowel syndrome, and postural orthostatic tachycardia syndrome. These are not separate silos. They may represent different expressions of the same underlying dysregulation involving mast cells, immune imbalance, and nervous system hypersensitivity(15). One example that often gets overlooked is costochondritis a painful condition that commonly shows up alongside fibromyalgia and is often mistaken for heart or lung-related pain. For many, this shift in understanding can be profound. It allows us to move away from a narrow pain-based model and toward a more integrated view of what’s actually going on. Not scattered symptoms, but a pattern. Not coincidence, but correlation.
Diagnostic Approaches Current and Emerging
Getting a diagnosis for fibromyalgia is hard enough. Adding mast cell activation into the mix makes it even more complicated. But that’s exactly why we need to look at the way both conditions are currently diagnosed and where that process might be falling short.
MCAS does not have a single test. Diagnosis usually depends on three things: a patient’s symptoms, lab evidence of mast cell mediators, and a positive response to mast cell-targeted treatment. Even with that framework, many people fall through the cracks. Symptoms can be vague, testing is time-sensitive, and results are often inconsistent(5).
One of the main lab markers used is serum tryptase, which reflects mast cell burden. In mastocytosis, tryptase is often elevated. In MCAS, it is usually normal unless measured during a flare. This means someone can have clear symptoms of mast cell activation and still receive a negative result(5). Other markers like chromogranin A, histamine, and prostaglandin D2 are also highly variable and require strict handling protocols. Many clinics are not equipped to run these tests accurately.
The situation with fibromyalgia is similar. Diagnosis still relies on clinical criteria, with no reliable biomarker. But that may soon change.
Infrared microspectroscopy, a specialised blood analysis technique, has been shown to distinguish fibromyalgia patients from those with rheumatoid arthritis or osteoarthritis with impressive accuracy. This method identifies distinct metabolic signatures, especially around tryptophan metabolism, which is heavily influenced by immune and nervous system activity(16).
Separately, a 2025 machine learning study identified three genes called DYRK3, RGS17, and ARHGEF37 that were consistently altered in fibromyalgia patients. These genes are involved in immune regulation and cellular signalling. The study also reported significant differences in mast cell numbers and immune cell composition, lending further support to the idea that fibromyalgia has an immunological footprint(17).
At the moment, most people with fibromyalgia or MCAS are still diagnosed by recognising patterns rather than pinpointing specific test results. But the gap between what science can detect and what clinicians use is beginning to close. New tools are coming. Better tests are on the horizon.
And for those who have spent years chasing answers, that progress matters. It suggests we are heading toward a future where diagnosis is no longer based on exclusion, but on recognition. Not a last-resort label, but a clearly defined condition with its own measurable biology.
Treatment Implications: A New Therapeutic Path
Understanding the role of mast cells in fibromyalgia changes the conversation. Instead of focusing only on symptom suppression, it opens the door to addressing one of the potential underlying drivers of the condition. That shift brings both challenges and possibilities.
Several studies have explored the use of mast cell stabilisers in animal models. In rats showing fibromyalgia like symptoms, ketotifen reduced pain responses and improved muscle function. Although this medication is commonly used in allergic conditions, its ability to calm overactive mast cells makes it a compelling candidate for broader use(8). Human trials are still limited, but the early data offer a strong basis for further investigation.
Antihistamines, especially those targeting both H1 and H2 receptors, may also help reduce mast cell driven symptoms. These are already widely available and have shown anecdotal benefits for people with fibromyalgia like presentations that include flushing, nausea, or cognitive fog. For some, combining them with stabilisers or anti-leukotriene medications may offer broader relief. That said, responses vary, and trialling these approaches should always be done with professional guidance. For those looking to reintroduce movement safely, we’ve written a guide on how to approach exercise with fibromyalgia in a way that supports recovery rather than aggravates symptoms.
There is also growing interest in dietary strategies that reduce histamine load or stabilise the gut environment. While evidence is still emerging, some individuals report improvements on low histamine, low FODMAP, or gluten-free diets. These approaches may reduce exposure to mast cell triggers and help calm systemic reactivity. It is not about restriction for the sake of it. It is about removing stressors that can provoke immune and neurological reactions.
Newer approaches include the use of repurposed medications. As mentioned earlier, modafinil has shown promise in animal models by interrupting the inflammatory pathways linked to mast cell activation. Although not yet approved for this use in humans, the findings suggest that medications which influence both immune and neurological signalling may hold potential for fibromyalgia in the future(7). One example of this in practice is low-dose naltrexone, which has been gaining attention for its off-label use in fibromyalgia.
There’s also research looking at less conventional ways of calming mast cell activity. One particularly interesting study found that exposing isolated rat mast cells to 100 percent carbon dioxide almost completely prevented degranulation. Not only did histamine release drop to baseline levels, but the calcium signals that usually trigger mast cell activation were suppressed by over seventy percent(18). This wasn’t achieved through normal breathing, it involved direct cellular exposure in a lab setting — but it has sparked interest in intranasal CO2 therapies already used for allergic rhinitis. At this stage, any application for fibromyalgia or MCAS remains theoretical, but it’s a fascinating direction for future treatment development. For a more grounded, accessible option, outdoor activity has also been shown to benefit mood, inflammation, and pain sensitivity in fibromyalgia.
Importantly, none of these strategies offer a universal solution. What works for one person may not work for another. That is why a personalised approach matters. Recognising mast cell involvement helps to create a clearer framework for testing, experimentation, and adaptation, rather than relying on trial and error alone.
Most importantly, it gives people something they have often lacked in the past. A way to understand their symptoms that is grounded in biology. And with that understanding comes a chance to target the condition more effectively and more compassionately.
Where Do We Go From Here
As research continues to build, the mast cell connection offers a new way of thinking about fibromyalgia. But turning insight into impact takes time. The gap between discovery and clinical application remains wide, and many healthcare professionals are not yet trained to recognise or address mast cell involvement.
That said, change is happening. New testing methods are being developed. More researchers are beginning to explore fibromyalgia as a complex interplay between immune and neurological systems rather than a purely pain-processing disorder.
For individuals living with this condition, this shift can be both validating and practical. It offers a new framework for understanding symptoms that were once dismissed. It also points to treatment options that extend beyond antidepressants or pain modulation.
But caution still matters. Not every fibromyalgia case involves mast cell activation. Not every strategy will work the same way for everyone. This is where thoughtful clinical care becomes essential. Working with practitioners who understand the complexity of the condition and who are open to immune based mechanisms can make a significant difference.
It is also where self advocacy plays a role. Asking questions. Tracking symptoms. Noticing patterns. These are not just helpful strategies. They are often what guide people to the right answers when systems fall short.
So where do we go from here? We follow the evidence. We keep asking better questions. And we continue to push for care that reflects the full reality of what people are experiencing.
For those who have spent years being told their test results are normal, or that it is all in their head, this new perspective offers something powerful. Not just information, but direction.
Hope Through Understanding
For years, fibromyalgia has existed in a grey area. A condition with real symptoms but no clear explanation. A diagnosis that often came with more questions than answers. But as the research grows, so does our understanding. Mast cells are no longer a fringe theory. They are a legitimate piece of the puzzle for many people living with this condition.
This is not about reinventing fibromyalgia. It is about refining it. About recognising that immune signalling, neurological sensitivity, and systemic inflammation can all be part of the same story. A story that has been unfolding quietly in the background for far too long.
If you have felt unheard, dismissed, or confused by your symptoms, you are not alone. The patterns you have noticed in your own body have a basis in science. And while the road to better care may still have barriers, it is now paved with clearer directions.
Knowledge is not a cure. But it is a beginning. It allows you to make more informed choices. To seek care that fits your experience. And to find a sense of validation in what your body has been telling you all along.
Have you seen yourself in this? Do these patterns sound familiar? We would love to hear about your experience in the comments.
You deserve more than symptom management. You deserve answers.

References
- Theoharides, T. C. (2021). Could mast cells be the missing link in fibromyalgia? Clinical Therapeutics, 43(8), 1225–1229. https://doi.org/10.1016/j.clinthera.2021.06.004
- Theoharides, T. C., & Kavalioti, M. (2021). Mast cells, stress, fear and pain. Journal of Biological Regulators & Homeostatic Agents, 35(2), 343–347.
- Goebel, A., et al. (2021). Passive transfer of fibromyalgia symptoms from patients to mice. The Journal of Clinical Investigation, 131(13). https://doi.org/10.1172/JCI144201
- Vaeroy, H., et al. (1988). Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis. Pain, 32(1), 21–26. https://doi.org/10.1016/0304-3959(88)90004-6
- Afrin, L. B., Weinstock, L. B., & Molderings, G. J. (2016). The presentation, diagnosis, and management of mast cell activation syndrome. Current Treatment Options in Allergy, 3(3), 350–364. https://doi.org/10.1007/s40521-016-0092-7
- Subramanian, H., Gupta, K., & Ali, H. (2016). Roles of Mas-related G protein–coupled receptor X2 on mast cell–mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. Journal of Allergy and Clinical Immunology, 138(3), 700–710. https://doi.org/10.1016/j.jaci.2016.06.009
- Zuo, J., et al. (2025). Modafinil alleviates fibromyalgia-like symptoms in rats by regulating the SP/MRGPRX2/IL-17 axis. Neuroscience Letters, 781, 137004.
- Li, W., et al. (2020). Ketotifen fumarate attenuates fibromyalgia-like symptoms by suppressing mast cell activation in a reserpine-induced fibromyalgia model. International Immunopharmacology, 88, 106943. https://doi.org/10.1016/j.intimp.2020.106943
- Albrecht, D. S., et al. (2019). Brain glial activation in fibromyalgia – A multi-site positron emission tomography investigation. Brain, Behavior, and Immunity, 75, 72–83. https://doi.org/10.1016/j.bbi.2018.09.018
- Loggia, M. L., et al. (2015). Evidence for brain glial activation in chronic pain patients. Brain, 138(3), 604–615. https://doi.org/10.1093/brain/awu377
- Üçeyler, N., Häuser, W., & Sommer, C. (2011). Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskeletal Disorders, 12, 245. https://doi.org/10.1186/1471-2474-12-245
- Clos-Garcia, M., et al. (2019). Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine, 46, 499–511. https://doi.org/10.1016/j.ebiom.2019.07.005
- Maintz, L., & Novak, N. (2007). Histamine and histamine intolerance. The American Journal of Clinical Nutrition, 85(5), 1185–1196. https://doi.org/10.1093/ajcn/85.5.1185
- Castori, M., et al. (2015). Chronic pain in Ehlers-Danlos syndrome: insights into mechanisms, management, and future directions. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 169(1), 1–7. https://doi.org/10.1002/ajmg.c.31427
- Oaklander, A. L., & Klein, M. M. (2013). Evidence of small-fiber polyneuropathy in unexplained, juvenile-onset widespread pain syndromes. Pediatrics, 131(4), e1091–e1100. https://doi.org/10.1542/peds.2012-2597
- Hackshaw, K. V., et al. (2013). Evidence for uric acid and purine metabolism pathway alterations in the serum of women with fibromyalgia. Clinical and Translational Science, 6(4), 280–285. https://doi.org/10.1111/cts.12068
- Li, Y., et al. (2025). Machine learning identifies immune cell changes and novel gene signatures in fibromyalgia. Frontiers in Immunology, 16, 1123347. https://doi.org/10.3389/fimmu.2025.1123347
- Strider, J.W., Masterson, C.G. and Durham, P.L., 2011. Treatment of mast cells with carbon dioxide suppresses degranulation via a novel mechanism involving repression of increased intracellular calcium levels. Allergy, 66(3), pp.341-350.